Authors:
Luczywo, Ayelen [1] ; Gonzalez, Lucia G. [1] ; Aguiar, Anna C. C. [2] ; Oliveira de Souza, Juliana [2] ; Souza, Guilherme E. [2] ; Oliva, Glaucius [2] ; Aguilar, Luis F. [3] ; Casal, Juan J. [1, 4] ; Guido, Rafael V. C. [2] ; Asis, Silvia E. [1] ; Mellado, Marco [3]
Abstract:
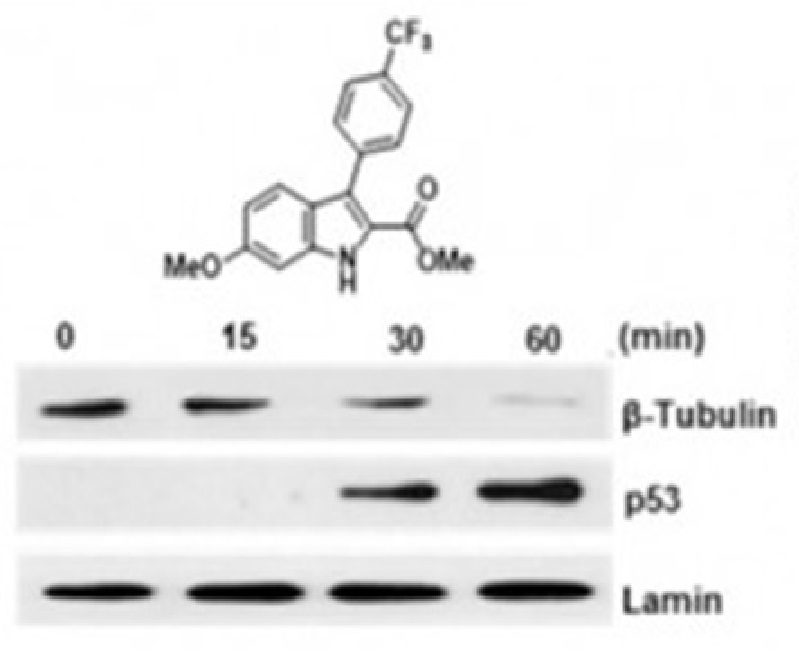
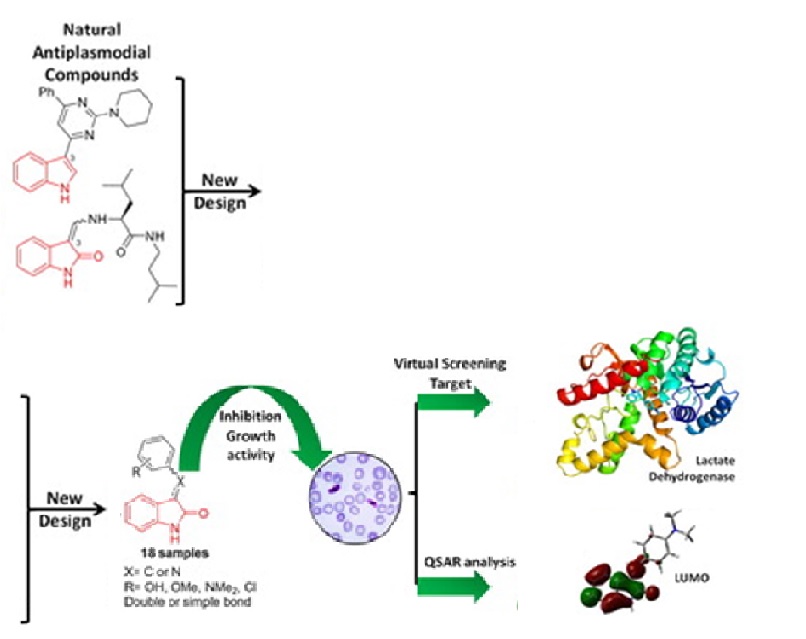
Malaria is an infectious illness, affecting vulnerable populations in Third World countries. Inspired by natural products, indole alkaloids have been used as a nucleus to design new antimalarial drugs. So, eighteen oxindole derivatives, aza analogues were obtained with moderate to excellent yields. Also, the saturated derivatives of oxindole and aza derivatives via H2/Pd/C reduction were obtained in good yields, leading to racemic mixtures of each compound. Next, the inhibitory activity against P. falciparum of 18 compounds were tested, founding six compounds with IC50 < 20 µM. The most active of these compounds was 8c; however, their unsaturated derivative 7c was inactive. Then, a structure-activity relationship analysis was done, founding that focused LUMO lobe on the specific molecular zone is related to inhibitory activity against P. falciparum. Finally, we found a potential inhibition of lactate dehydrogenase by oxindole derivatives, using molecular docking virtual screening.
1 Departamento de Química Orgânica , Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina
2 Instituto de Física de São Carlos, Univiersidade de São Paulo, São Carlos, Brasil
3 Instituto de Quimica, , Pontifícia Universidad Católica de Valparaiso, Valparaiso, Chile
4 Facultad de Medcina, Laboratorio de Biomembranas, Instituto de Fisiologa y Biofísica Bernardo Houssay (IFIO Houssay) , Universidad de Buenos Aires-CONICET, Buenos Aires, Argentina
Link to article: https://www.tandfonline.com/doi/full/10.1080/14786419.2021.1895149