Authors:
de Freitas, Larissa [1] ; Valli, Marilia [1, 2] ; Dametto, Alessandra C. [1, 3] ; Pennacchi, Paula C. [4] ; Andricopulo, Adriano D. [2] ; Maria-Engler, Silvya S. [4] ; Bolzani, Vanderlan S. [1]
Abstract:
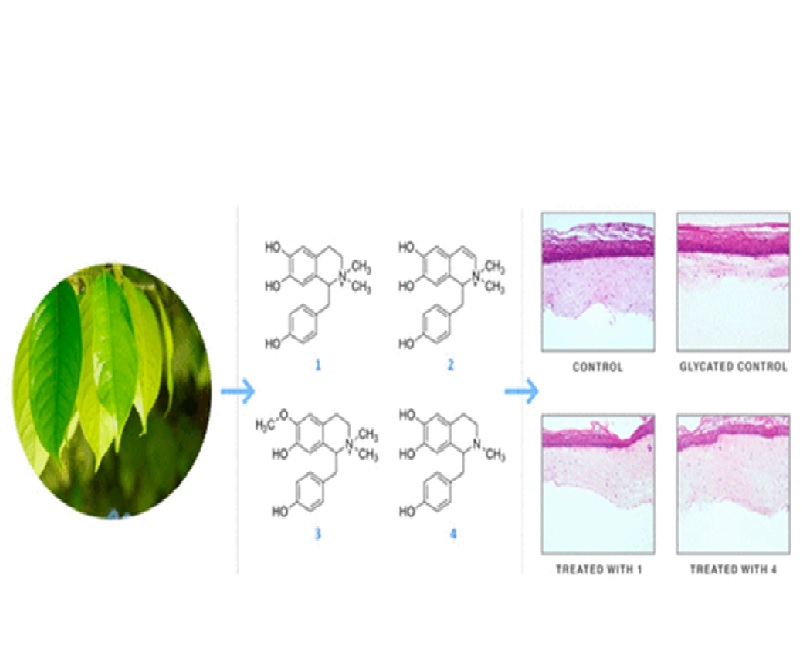
A bioassay-guided study aiming at identifying inhibitors of the glycation process on the leaves of Ocotea paranapiacabensis afforded four benzylisoquinoline alkaloids (1-4), with 1 and 2 identified as new naturals products, while 3 and 4 were previously described in the literature, with 3 being identified as magnocurarine. Purification was performed by column chromatography and high-performance liquid chromatography. The structures of the isolated compounds were elucidated by spectroscopic methods including UV, NMR, and HRMS. The process of skin aging has been recently associated with advanced glycation end products (AGEs), and strategies inhibiting their formation have been addressed by pharmaceutical companies for the development of novel antiaging compounds. Alkaloids 1-4 were evaluated for their potential to inhibit AGE formation and showed inhibition of 62.9%, 83.3%, 26.1%, and 98.2% (150 mu M), respectively. The antiaging potential of compounds 1 and 4 were evaluated with a reconstructed human skin model in vitro, and results showed a decrease in dermis contraction (8.7% and 4.2% respectively for 1 and 4) when compared to the glycated control (57.4%). Additionally, absorption, distribution, metabolism, and excretion (ADME) and toxicity properties were predicted using in silico methods, and the results were considered significantly promising for alkaloids 1 and 4 to continue the development of these alkaloids with skincare properties.
1 Nuclei of Bioassays, Biosynthesis and Ecophysiology of Natural Products (NuBBE), Department of Organic Chemistry, Institute of Chemistry, São Paulo State University (UNESP), Avenida Prof. Francisco Degni, 55, Araraquara, SP, Brazil
2 Laboratory of Medicinal and Computational Chemistry (LQMC), Centre for Research and Innovation in Biodiversity and Drug Discovery (CIBFar), Institute of Physics of São Carlos, University of São Paulo (USP), Avenida João Dagnone, no. 1100, São Carlos, SP, Brazil
3 Federal Institute of Education, Science and Technology of São Paulo, Rua Stéfano D’avassi, no. 625, Matão, SP, Brazil
4 Department of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, University of São Paulo (USP), São Paulo, Brazil
Link to article: https://pubs.acs.org/doi/10.1021/acs.jnatprod.9b01083