Autores:
Vieira, Lucas C. C.1 2 ; Matsuo, Bianca T.1 ; Martelli, Lorena S. R.1 ; Gall, Mayara1 ; Paixao, Marcio W.1 ; Correa, Arlene G.1
Resumo:
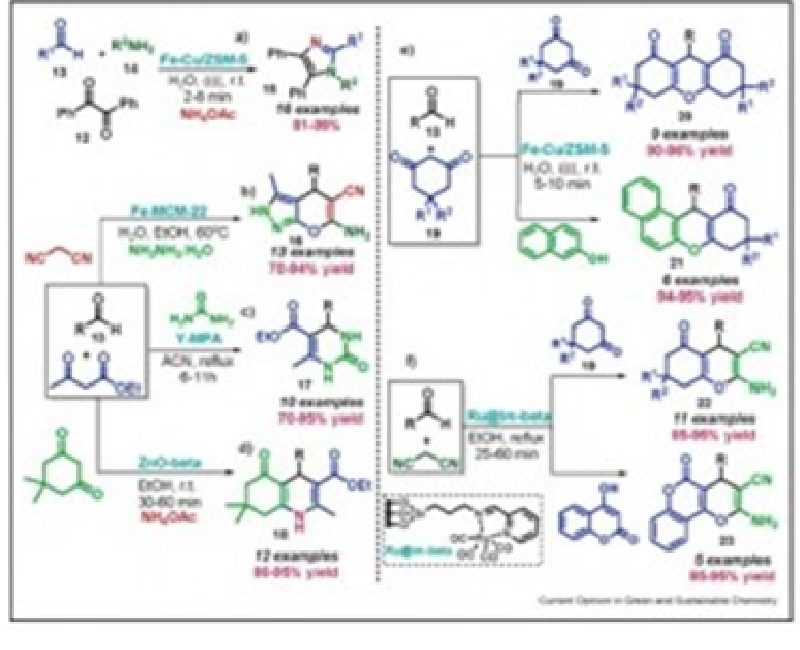
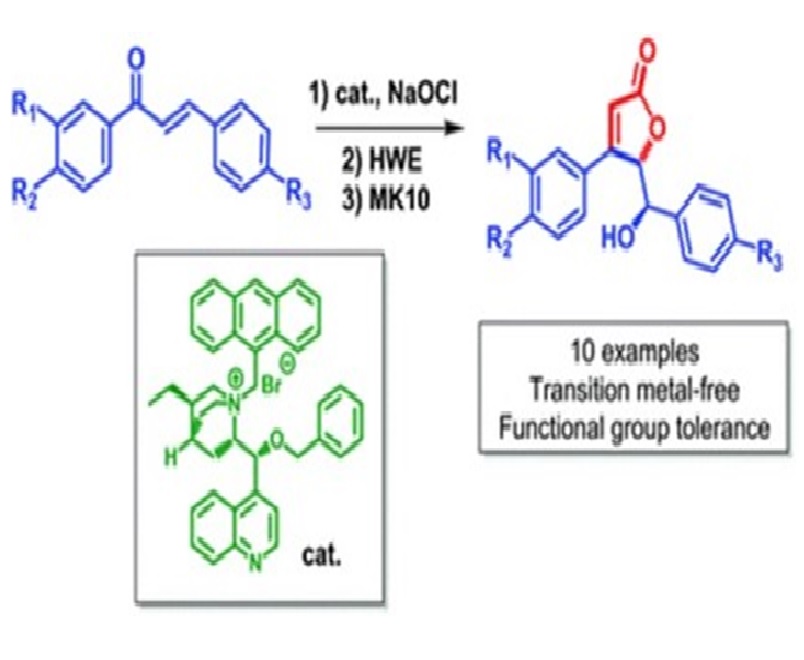
γ-Butenolides have been recognized as an important structural framework in a number of natural products and medicinally important agents. In this work we describe a new metal-free sequential strategy for the asymmetric synthesis of substituted γ-butenolides having epoxychalcones as the advanced intermediate. Using the optimized reaction conditions, we were able to carry out the three-step sequence, epoxidation, olefination and hydrolysis, with only one single chromatographic purification of the final product, furnishing new enantiomerically enriched γ-butenolides in moderate overall yield and good enantiomeric excess.
1 Centre of Excellence for Research in Sustainable Chemistry, Department of Chemistry, Federal University of São Carlos, São Carlos, Brazil
2 Instituto de Engenharia, Universidade Federal de Mato Grosso, 78060-900, Cuiabá, Brazil
Link to full article:
http://pubs.rsc.org/en/Content/ArticleLanding/2017/OB/C7OB00165G#!divAbstract