Authors:
Silva, Thiago S. [1] ; Zeoly, Lucas A. [1] ; Coelho, Fernando [1]
Abstract:
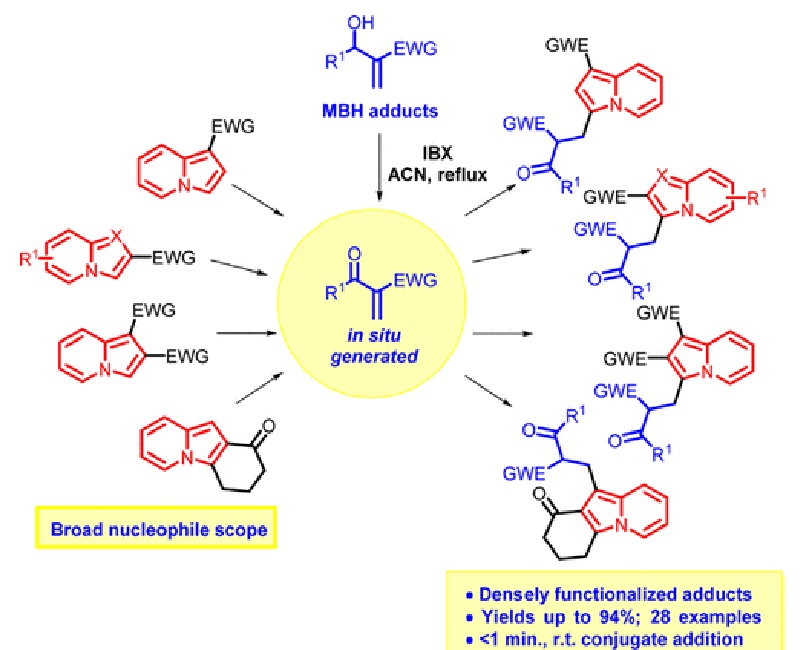
Sequential one-pot 2-iodoxybenzoic acid (IBX) oxidation of Morita-Baylis-Hillman (MBH) adducts followed by catalyst-free indolizine conjugate addition was developed. The wide scopes of MBH adducts and indolizines were investigated, and densely functionalized adducts were obtained in yields of up to 94%. The conjugate addition step occurred in less than a minute at room temperature with total regioselectivity toward indolizine C3 carbon. Less nucleophilic C1 carbon was also alkylated when C3-substituted indolizines were employed as the substrate.
1 Laboratory of Synthesis of Natural Products and Drugs, Institute of Chemistry, University of Campinas, UNICAMP, P.O. Box 6154, 13083-970, Campinas, SP, Brazil
Link to article: https://pubs.acs.org/doi/abs/10.1021/acs.joc.0c00189