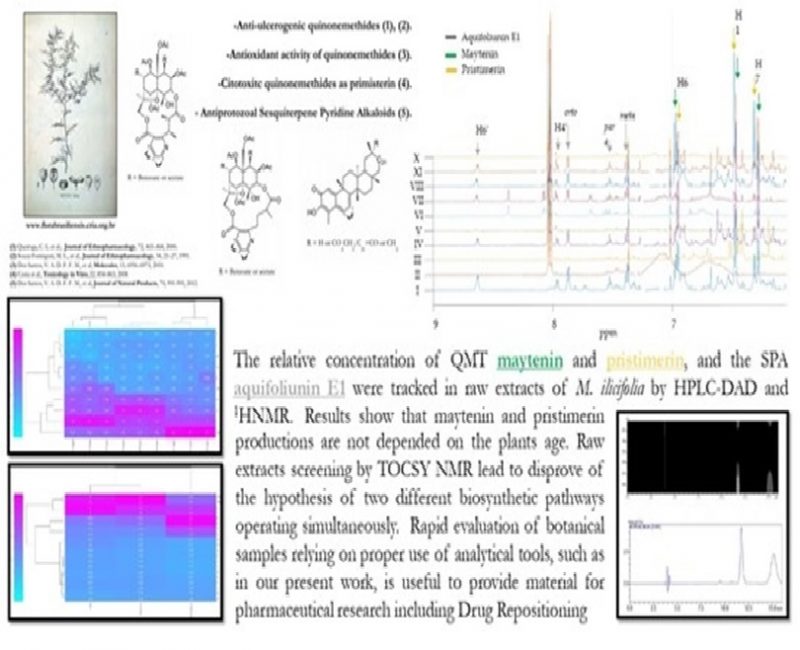
Authors:
Silva, Rodrigo Moreira da 1- 2 ; Gaitani, Cristiane Masetto de 2 ; Marques, Lucas Maciel Mauriz 1 ; Baraco, Karina Fraige 3 ; Cavalheiro, Alberto José 3 ; Moraes, Luiz Alberto Beraldo de 4 ; Lopes, Norberto Peporine 1 ; Oliveira, Anderson Rodrigo Moraes de 4 ;
Abstract:
Casearin X (CAS X) is the major clerodane diterpene isolated from the leaves of Casearia sylvestris and has been extensively studied due to its powerful cytotoxic activity at low concentrations. Promising results for in vivo antitumor action have also been described when CAS X was administered intraperitoneally in mice. Conversely, loss of activity was observed when orally administered. Since the advancement of natural products as drug candidates requires satisfactory bioavailability for their pharmacological effect, this work aimed to characterize the CAS X metabolism by employing an in vitro microsomal model for the prediction of preclinical pharmacokinetic data. Rat and human liver microsomes were used to assess species differences. A high-performance liquid chromatography with diode-array detection (HPLC-DAD) method for the quantification of CAS X in microsomes was developed and validated according to European Medicines Agency guidelines. CAS X was demonstrated to be a substrate for carboxylesterases via hydrolysis reaction, with a Michaelis-Menten kinetic profile. The enzyme kinetic parameters were determined, and the intrinsic clearance was 1.7-fold higher in humans than in rats. The hepatic clearance was estimated by in vitro-in vivo extrapolation, resulting in more than 90% of the hepatic blood flow for both species. A qualitative study was also carried out for the metabolite identification by mass spectrometry and indicated the formation of the inactive metabolite CAS X dialdehyde. These findings demonstrate that CAS X is susceptible to first-pass metabolism and is a substrate for specific carboxylesterases expressed in liver, which may contribute to a reduction in antitumor activity when administered by the oral route.
1 Núcleo de Pesquisa em Produtos Naturais e Sintéticos (NPPNS), Departamento de Física e Química, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil
2 Departamento do Ciências Farmacêuticas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil
3 Núcleo de Bioensaios, Biossíntese e Ecofisiologia de Produtos Naturais (NuBBE), Departamento de Química Orgânica, Instituto de Química, Universidade do Estado de São Paulo, Araraquara, SP, Brazil
4 Departamento de Química, Faculdade de Filosofia Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil
Link to article: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/a-0765-9523