Authors:
Kattela, Shivashankar 1 ; de Lucca Jr, Emilio C. 1 ; Correia, Carlos Roque D. 1
Abstract:
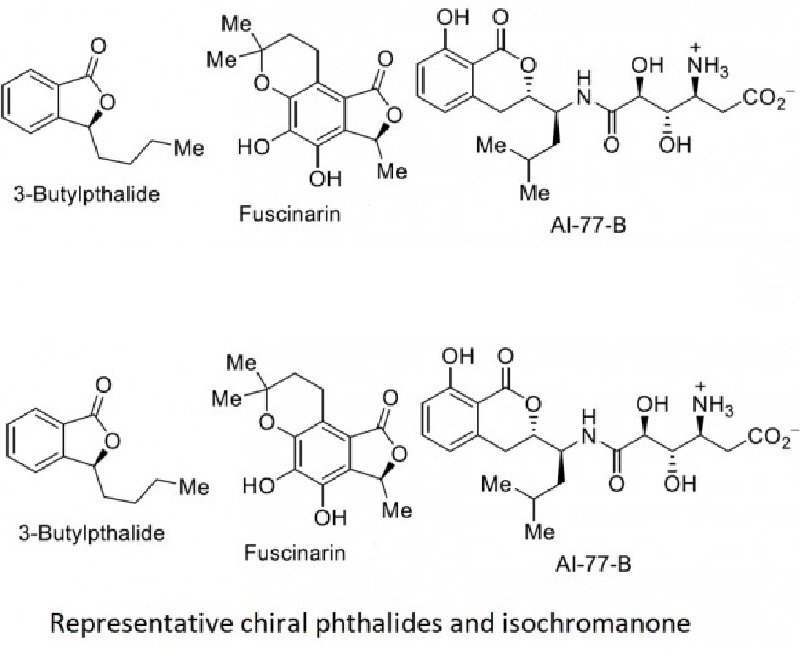
In this communication, we describe the enantioselective synthesis of phthalides and isochromanones through a new palladium-catalyzed Heck–Matsuda arylation/NaBH4-reduction/lactonization sequenceof 2,3- and 2,5-dihydrofurans in good overall yields and excellent enantioselectivities (up to 98:2 er). Thisexpeditious synthesis of chiral Heck lactol intermediates allowed thediversification of the strategy to obtain medicinally relevant chiral lactones, amines, and olefins. The natural product 3-butylphthalide was obtained in 3 steps with an overall yield of 33% yield in 98:2 er.
1 University of Campinas, Institute of Chemistry, 10384-612, São Paulo (Brazil)
Link to full article: https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.20180495824