Autores:
Masias, Emilse 1; Dupuy, Fernando G.1 ; da Silva Sanches, Paulo Ricardo2 ; Farizano, Juan Vicente1 ; Cilli, Eduardo2 ; Bellomio, Augusto1 ; Saavedra, Lucila3 ; Minahk, Carlos1
Resumo:
Background
Enterocin CRL35 is a class IIa bacteriocin with anti-Listeria activity. Resistance to these peptides has been associated with either the downregulation of the receptor expression or changes in the membrane and cell walls. The scope of the present work was to characterize enterocin CRL35 resistant Listeria strains with MICs more than 10,000 times higher than the MIC of the WT sensitive strain.
Methods
Listeria monocytogenes INS7 resistant isolates R2 and R3 were characterized by 16S RNA gene sequencing and rep-PCR. Bacterial growth kinetic was studied in different culture media. Plasma membranes of sensitive and resistant bacteria were characterized by FTIR and Langmuir monolayer techniques.
Results
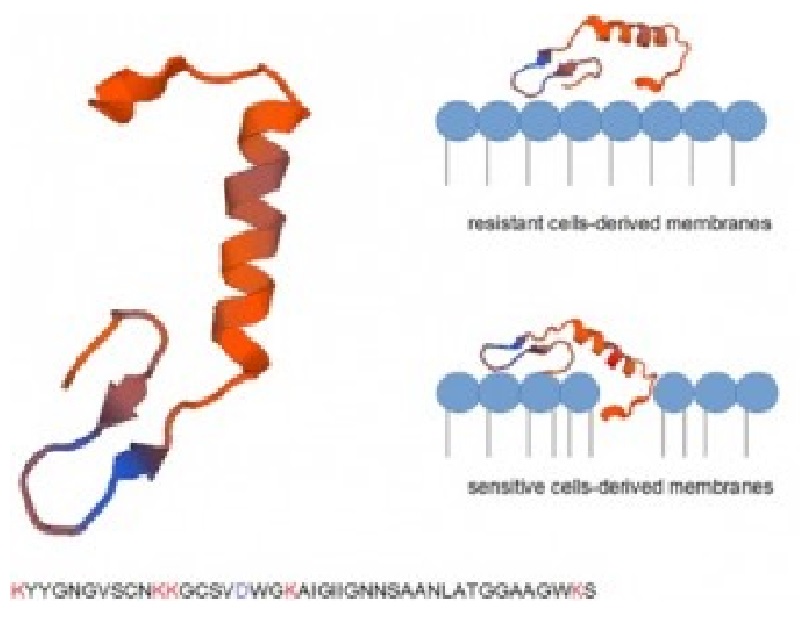
The growth kinetic of the resistant isolates was slower as compared to the parental strain in TSB medium. Moreover, the resistant isolates barely grew in a glucose-based synthetic medium, suggesting that these cells had a major alteration in glucose transport. Resistant bacteria also had alterations in their cell wall and, most importantly, membrane lipids. In fact, even though enterocin CRL35 was able to bind to the membrane-water interface of both resistant and parental sensitive strains, this peptide was only able to get inserted into the latter membranes.
Conclusions
These results indicate that bacteriocin receptor is altered in combination with membrane structural modifications in enterocin CRL35-resistant L. monocytogenes strains.
General significance
Highly enterocin CRL35-resistant isolates derived from Listeria monocytogenes INS7 have not only an impaired glucose transport but also display structural changes in the hydrophobic core of their plasma membranes.
1 Instituto Superior de Investigaciones Biológicas (INSIBIO), CONICET-UNT and Instituto de Química Biológica “Dr. Bernabé Bloj”, Facultad de Bioquímica, Química y Farmacia, UNT, Chacabuco 461, T4000ILI San Miguel de Tucumán, Argentina
2 Departamento de Bioquímica e Tecnologia Química, Instituto de Química, UNESP-Univ Estadual Paulista, Araraquara, SP, Brazil
3 Centro de Referencia para Lactobacilos, Chacabuco 145, San Miguel de Tucumán, Argentina
Link to full article:
http://www.sciencedirect.com/science/article/pii/S0304416517300971?via%3Dihub