Authors:
Martelli, Lorena S. R. 1 ; Vieira, Lucas C. C. 1, 2 ; Paixao, Marcio W. 1 ; Zukerman-Schpector, Julio 1 ; de Souza, Juliana O. 3 ; Aguiar, Anna Caroline C. 3 ; Oliva, Glaucius 3 ; Guido, Rafael V. C. 3 ; Correa, Arlene G. 1
Abstract:
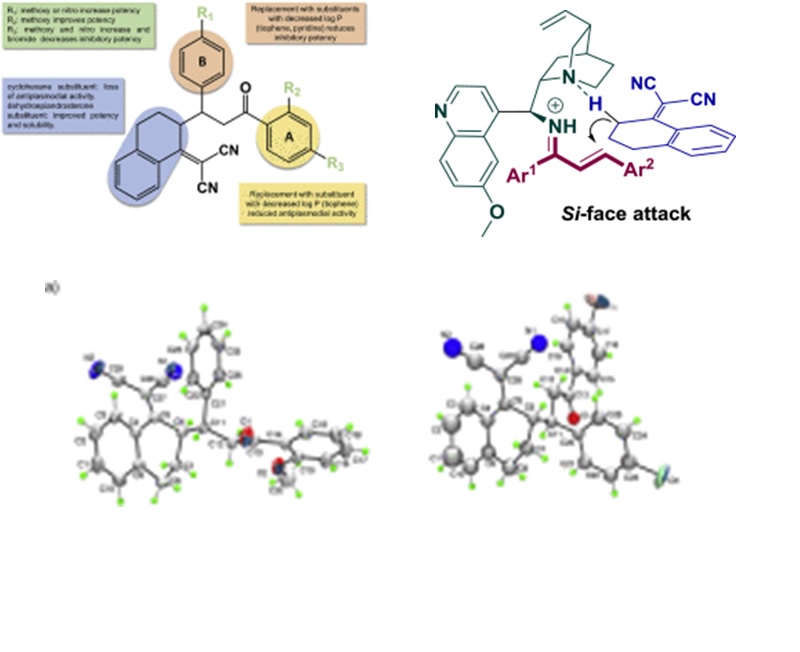
The organocatalysed asymmetric vinylogous Michael addition of α,α-dicyanoolefins to α,β-unsaturated aldehydes and ketones have been reported in the last decade, however, chalcones have been poorly explored. Moreover, a considerable part of the publications in this theme still employs undesirable solvents, such as toluene and THF, with concerns related to health and environmental safety. We report herein the use of a bifunctional catalyst derived from a Cinchona alkaloid to perform the enantio- and diastereoselective Michael addition of α,α-dicyanoolefins to chalcones using 2-MeTHF as solvent. The Michael adducts were obtained in moderate to good yields and were evaluated for their antiplasmodial and cytotoxic activity.
1 Centre of Excellence for Research in Sustainable Chemistry, Department of Chemistry, Federal University of São Carlos, 13565-905, São Carlos, SP, Brazil
2 Institute of Engineering, Federal University of Mato Grosso, 78060-900, Cuiabá, MT, Brazil
3 Center for Innovation in Biodiversity and Drug Discovery, São Carlos Institute of Physics, University of São Paulo, 13563-120, São Carlos, SP, Brazil
Link to article: https://www.sciencedirect.com/science/article/pii/S0040402019305617?via%3Dihub1