Authors:
da Silva, Erica C. 1 ; Yamakawa, Nathalia C. G. 1 ; Dos Santos, Alcindo A. 2 ; Coelho, Fernando 1
Abstract:
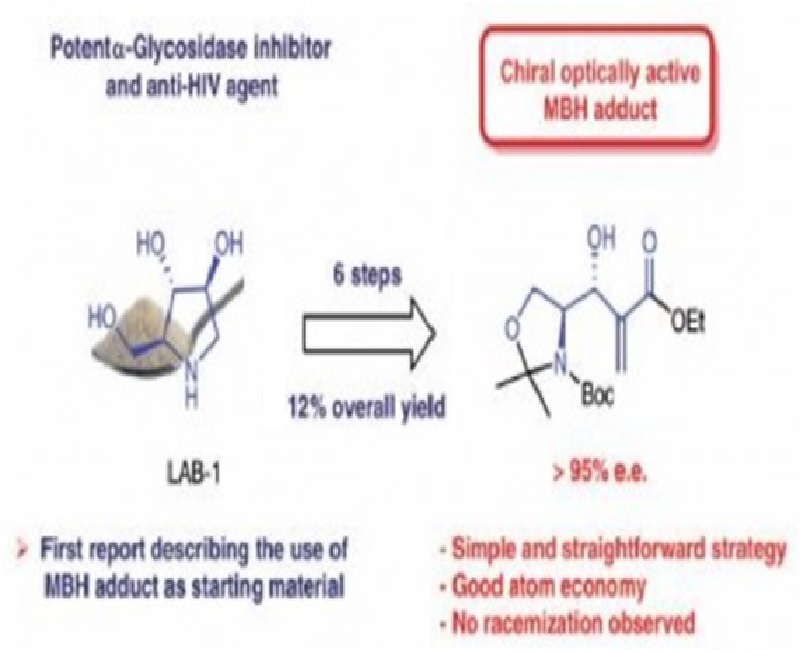
We described herein a total synthesis of 1,4-dideoxy-1,4-imino-l-arabinitol [(2S,3S,4S)-2-(hydroxymethyl)pyrrolidine-3,4-diol, LAB-1], a polyhydroxylated pyrrolidine, which has been demonstrated to be a selective and potent α-glycosidase inhibitor. The main features of our approach are its shortness, efficiency, and simplicity. The total synthesis was accomplished in 6 steps with an overall yield of 12%, starting from a chiral optically active Morita–Baylis–Hillman (MBH) adduct prepared (without epimerization), from Garner’s aldehyde. As far as we know, this is the first report describing the total synthesis of this biologically active pyrrolidine by exploring the synthetic versatility of a MBH adduct.
1 Univ Estadual Campinas, Inst Chem, Lab Synth Nat Prod & Drugs, POB 6154, BR-13083970 Campinas, SP – Brazil
2 Univ Sao Paulo, Inst Chem, Ave Prof Lineu Prestes, 748, Cidade Univ, BR-05508000 Sao Paulo, SP – Brazil
Link to full article: https://www.thieme-connect.de/DOI/DOI?10.1055/s-0036-1590799