Authors:
Fernandes, Fabio S. 1 ; Rodrigues, Jr., Manoel T. 1 ; Zeoly, Lucas A. 1 ; Conti, Caroline 1 ; Angolini, Celio F. F. 2 ; Eberlin, Marcos Nogueira 2, 3 ; Coelho, Fernando 1
Abstract:
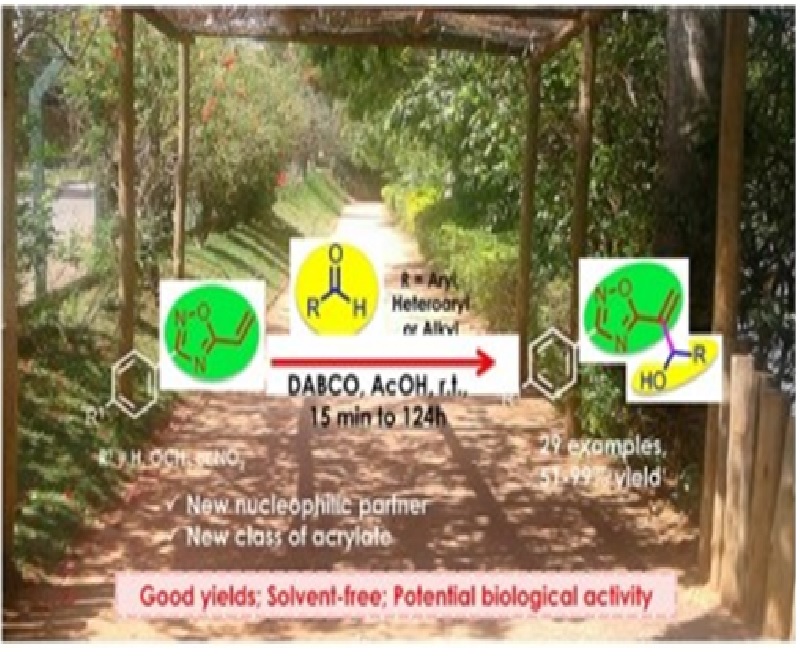
We describe that vinyl-oxadiazoles function as a new and efficient nucleophilic partner for the Morita–Baylis–Hillman (MBH) reaction. The reaction between 5-vinyl-3-aryl-1,2,4-oxadiazoles and aromatic and aliphatic aldehydes, catalyzed by DABCO in the absence of solvent, showed high efficiency to afford a new class of heterocyclic MBH adducts with potential biological activity on yields up to 99% and short reaction times. These synthetically attractive adducts bear a heterocyclic scaffold of large pharmaceutical and commercial interest associated with a plethora of biological effects and technological applications. We also demonstrate their synthetic usefulness by a photoinduced addition reaction to a polyfunctionalized amino alcohol.
1 Institute of Chemistry, Laboratório de Síntese de Produtos Naturais e Fármacos, POB 6154, BR-13083970 Campinas, SP – Brazil
2 Institute of Chemistry, Laboratório ThoMSon de Espectrometria de Massas, University of Campinas, P.O. Box 6154, Campinas, São Paulo 13083-970, Brazil
3 Mackenzie Presbiterian University, São Paulo, São Paulo 01302-907, Brazil
Link to article: