Authors:
Vicari, Hugo Passos [1] ; Lima, Keli [1] ; Gomes, Ralph da Costa [2] ; Fernandes, Daniara Cristina [3, 2] ; Lipreri da Silva, Jean Carlos [1] ; Rodrigues Junior, Manoel Trindade [2] ; Barroso de Oliveira, Aline Silva [2] ; dos Santos, Ricardo Nascimento [4] ; Andricopulo, Adriano Defini [4] ; Coelho, Fernando [2] ; Costa-Lotufo, Leticia Veras [1] ; Machado-Neto, Joao Agostinho [1]
Abstract:
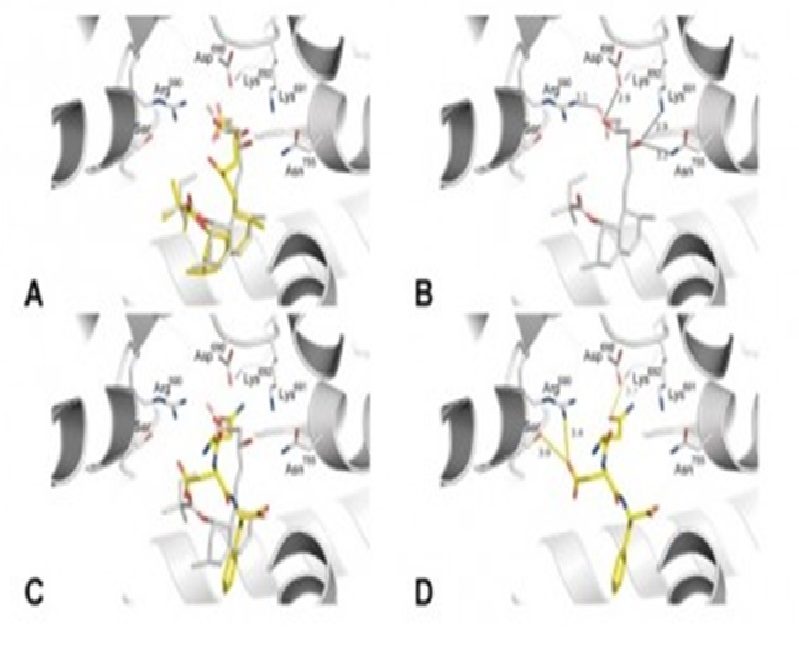
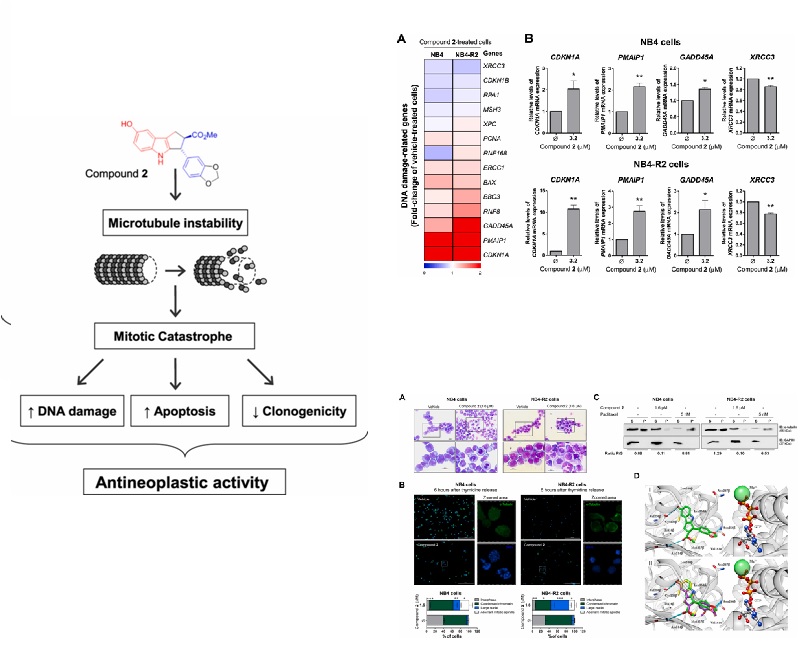
Acute promyelocytic leukemia (APL) is associated with PML-RAR alpha oncogene, which is treated using all-trans retinoic acid (ATRA)-based chemotherapy. However, chemoresistance is observed in 20-30% of treated patients and represents a clinical challenge, raising the importance of the development of new therapeutic options. In the present study, the effects of three synthetic cyclopenta{[}b]indoles on the leukemia phenotype were investigated using NB4 (ATRA-sensitive) and NB4-R2 (ATRA-resistant) cells. Among the tested synthetic cyclopenta{[}b]indoles, compound 2, which contains a heterocyclic nucleus, was the most active, presenting time-dependent cytotoxic activity in the mu M range in APL cells, without cytotoxicity for normal leukocytes, and was selected for further characterization. Compound 2 significantly decreased clonogenicity, increased apoptosis, and caused cell cycle arrest at S and G(2)/M phases in a drug concentration-dependent manner. Morphological analyses indicated aberrant mitosis and diffuse tubulin staining upon compound 2 exposure, which corroborates cell cycle findings. In the molecular scenario, compound 2 reduced STMN1 expression and activity, and induced PARP1 cleavage and H2AX and CHK2 phosphorylation, and modulated CDKN1A, PMAIP1, GADD45A, and XRCC3 expressions, indicating reduction of cell proliferation, apoptosis, and DNA damage. Moreover, in the in vivo tubulin polymerization assay, NB4 and NB4-R2 cells showed a reduction in the levels of polymerized tubulin upon compound 2 exposure, which indicates tubulin as a target of the drug. Molecular docking supports this hypothesis. Taken together, these data indicated that compound 2 exhibits antileukemic effects through disrupting the microtubule dynamics, identifying a possible novel potential antineoplastic agent for the treatment of ATRA-resistant APL.
1 Department of Pharmacology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, SP, 05508-900, Brazil
2 Department of Organic Chemistry, Chemistry Institute, University of Campinas, Campinas, São Paulo, SP, 13083-970, Brazil
3 Currently at Instituto Federal de Educação Ciência e Tecnologia de São Paulo, Matão, SP, 15991-502, Brazil
4 Physics Institute of São Carlos, University of São Paulo, São Carlos, SP, 13565-905, Brazil
Link to article: https://www.sciencedirect.com/science/article/abs/pii/S0014299921000066?via%3Dihub