Authors:
Medina, Rebeca P. 1 ; Araujo, Angela R. 1 ; Batista Jr, João M. 2, 3 ; Cardoso, Carmen L. 4 ; Seidl, Cláudia 4 ; Vilela, Adriana F. L. 4 ; Domingos, Helori V. 5 ; Costa-Lotufo, Leticia V. 5 ; Andersen, Raymond J. 6 ; Siqueira Silva, Dulce H.1 ;
Abstract:
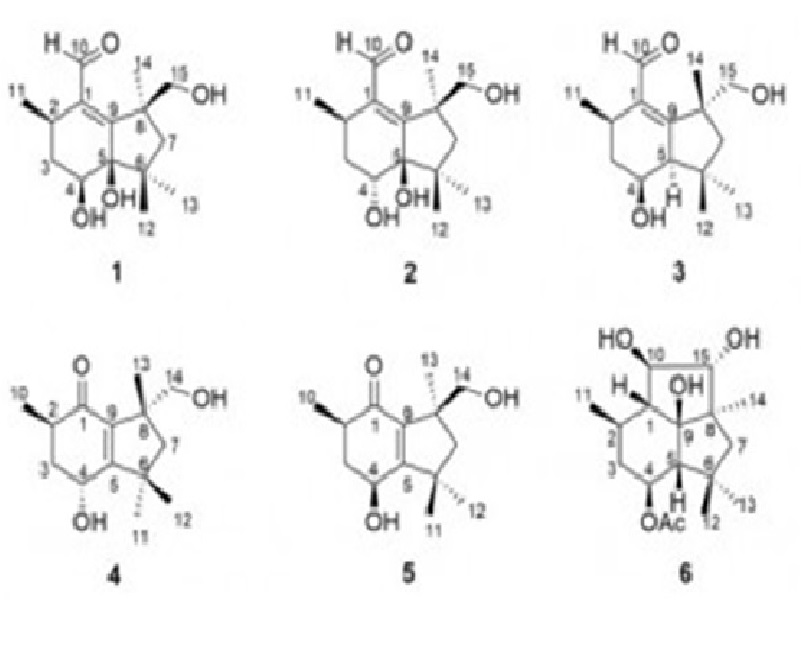
A chemical study of the EtOAc extract of Nemania bipapillata (AT-05), an endophytic fungus isolated from the marine red alga Asparagopsis taxiformis – Falkenbergia stage, led to the isolation of five new botryane sesquiterpenes, including the diastereomeric pair (+)-(2R,4S,5R,8S)-(1) and (+)-(2R,4R,5R,8S)-4-deacetyl-5-hydroxy-botryenalol (2), (+)-(2R,4S,5R,8R)-4-deacetyl-botryenalol (3), one pair of diastereomeric botryane norsesquiterpenes bearing an unprecedented degraded carbon skeleton, (+)-(2R,4R,8R)-(4) and (+)-(2R,4S,8S)-(5), which were named nemenonediol A and nemenonediol B, respectively, in addition to the known 4β-acetoxy-9β,10β,15α-trihydroxyprobotrydial (6). Their structures were elucidated using 1D and 2D NMR, HRESIMS and comparison with literature data of similar known compounds. The absolute configurations of 2, 3 and 4 were deduced by comparison of experimental and calculated electronic circular dichroism (ECD) spectra, while those of 1 and 5 were assigned from vibrational circular dichroism (VCD) data. Compound 4 weakly inhibited acetylcholinesterase, whereas compound 1 inhibited both acetylcholinesterase and butyrylcholinesterase. Compounds 1, 3, 5 and 6 were tested against two carcinoma cell lines (MCF-7 and HCT-116), but showed no significant citotoxicity at tested concentrations (IC50 > 50 µM).
1 Núcleo de Bioensaios, Biossíntese e Ecofisiologia de Produtos Naturais (NuBBE), Departamento de Química Orgânica, Instituto de Química, UNESP – Universidade Estadual Paulista, 14801-970, Araraquara-SP, Brazil
2 Departamento de Química, Centro de Ciências Exatas e de Tecnologia, Universidade Federal de São Carlos – UFSCar, 13565-905, São Carlos-SP, Brazil
3 Departamento de Ciência e Tecnologia, Universidade Federal de São Paulo –UNIFESP, 12231-280, São José dos Campos-SP, Brazil
4 Grupo de Cromatografia de Bioafinidade e Produtos Naturais, Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, 14040-901, Ribeirão Preto-SP, Brazil
5 Instituto de Ciências Biomédicas, Universidade de São Paulo, 05508-900, São Paulo-SP, Brazil
6 Departments of Chemistry and Earth, Ocean & Atmospheric Sciences, University of British Columbia, V6T 1Z1, Vancouver, BC, Canada
Link to article: https://www.nature.com/articles/s41598-019-48655-7#Abs1