Authors:
Polo, Ellen Christine 1 ; Wang, Marti Fernandez 1 ; Angnes, Ricardo Almir 2 ; Braga, Ataualpa A. C. 2 ; Duarte Correia, Carlos Roque 1
Abstract:
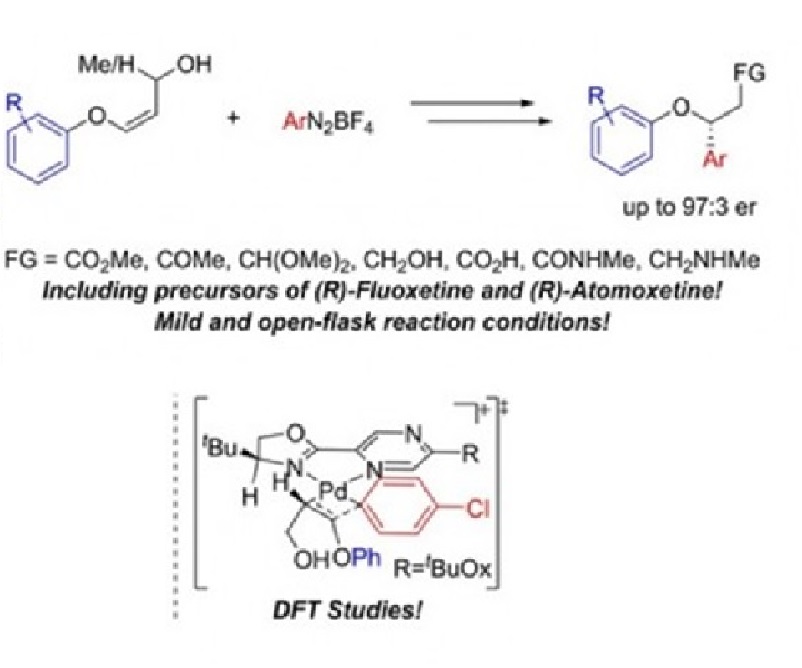
Herein we report the enantioselective Heck‐Matsuda arylation of acyclic E and Z‐alkenyl aryl ethers. The reactions were carried out under mild conditions affording the enantioenriched benzyl ethers in a regioselective manner, moderate to good yields (up to 73%), and in good to excellent enantiomeric ratios (up to 97:3). The enantioselective Heck‐Matsuda arylation has shown a broad scope (25 examples), and some key Heck‐Matsuda adducts were further converted into more complex and valuable scaffolds including their synthetic application in the synthesis of (R)‐Fluoxetine, (R)‐Atomoxetine, and in the synthesis of an enantioenriched benzo[c]chromene. Finally, in silico mechanistic investigations into the reaction’s enantioselectivity were performed using density functional theory.
1 Departamentode QuímicaOrgânica,Institutode Química,UniversidadeEstadualde Campinas,Rua Josué de Castro,s/n,13083-970,Campinas,São Paulo (Brazil)E-mail:croque@unicamp.br
2 Departamentode QuímicaFundamental,Institutode Química,Universidadede São Paulo, AvenidaLineu Prestes,748, 05508-000, São Paulo, São Paulo (Brazil)
Link to article: https://onlinelibrary.wiley.com/doi/full/10.1002/adsc.201901471