Authors:
Okada-Junior, Celso Yassuo 1 ; Monteiro, Gustavo Claro 1; Aguiar, Anna Caroline Campos 3 ; Batista, Victor Sousa 2 ; Souza, Juliana Oliveira de 3 ; Souza, Guilherme Eduardo 3 ; Bueno, Renata Vieira 3 ; Oliva, Glaucius 3 ; Nascimento-Júnior, Nailton M. 2 ; Guido, Rafael Victorio Carvalho 3 ; Bolzani, Vanderlan Silva 1
Abstract:
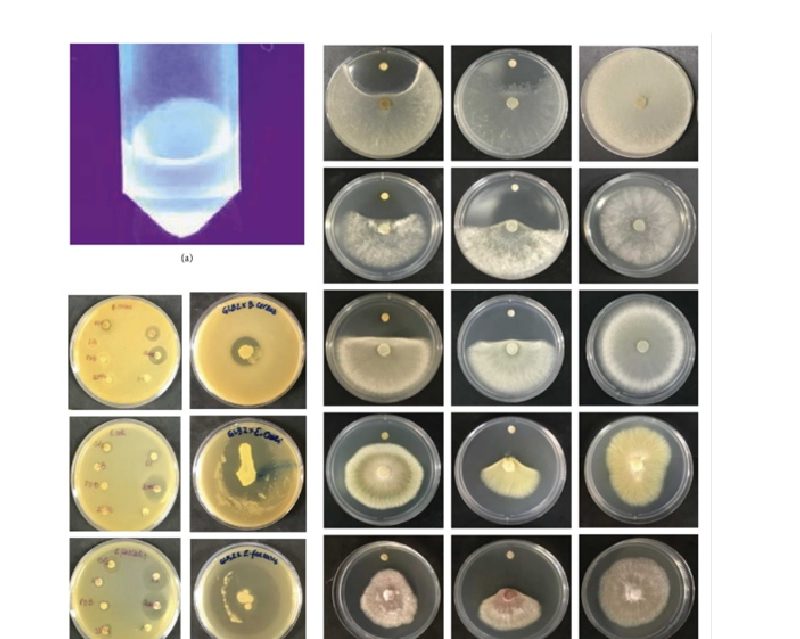
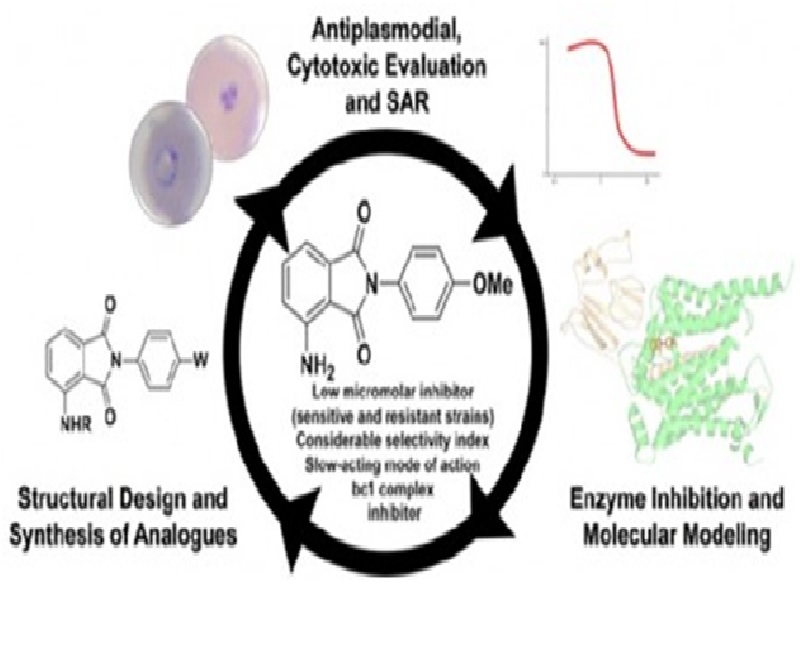
We describe herein the design and synthesis of N-phenyl phthalimide derivatives with inhibitory activities against Plasmodium falciparum (sensitive and resistant strains) in the low micromolar range and noticeable selectivity indices against human cells. The best inhibitor, 4-amino-2-(4-methoxyphenyl)isoindoline-1,3-dione (10), showed a slow-acting mechanism similar to that of atovaquone. Enzymatic assay indicated that 10 inhibited P. falciparum cytochrome bc1 complex. Molecular docking studies suggested the binding mode of the best hit to Qo site of the cytochrome bc1 complex. Our findings suggest that 10 is a promising candidate for hit-to-lead development.
1 Nucleus of Bioassays, Biosynthesis and Ecophysiology of Natural Products (NuBBE), Department of Organic Chemistry, Institute of Chemistry, São Paulo State University—UNESP, Rua Professor Francisco Degni, 55, Jardim Quitandinha, 14800-060 Araraquara, São Paulo, Brazil
2 Laboratory of Medicinal Chemistry, Organic Synthesis and Molecular Modeling (LaQMedSOMM), Department of Organic Chemistry, Institute of Chemistry, São Paulo State University—UNESP, Rua Professor Francisco Degni, 55, Jardim Quitandinha, 14800-060 Araraquara, São Paulo, Brazil
3 Sao Carlos Institute of Physics, University of Sao Paulo, Av. Joao Dagnone, 1100 Jardim Santa Angelina, Sao Carlos, São Paulo 13563-120, Brazil
Link para o artigo completo: https://pubs.acs.org/doi/abs/10.1021/acsomega.8b01062