Authors:
Lima, Samia R. [1] ; Coelho, Fernando [1]
Abstract:
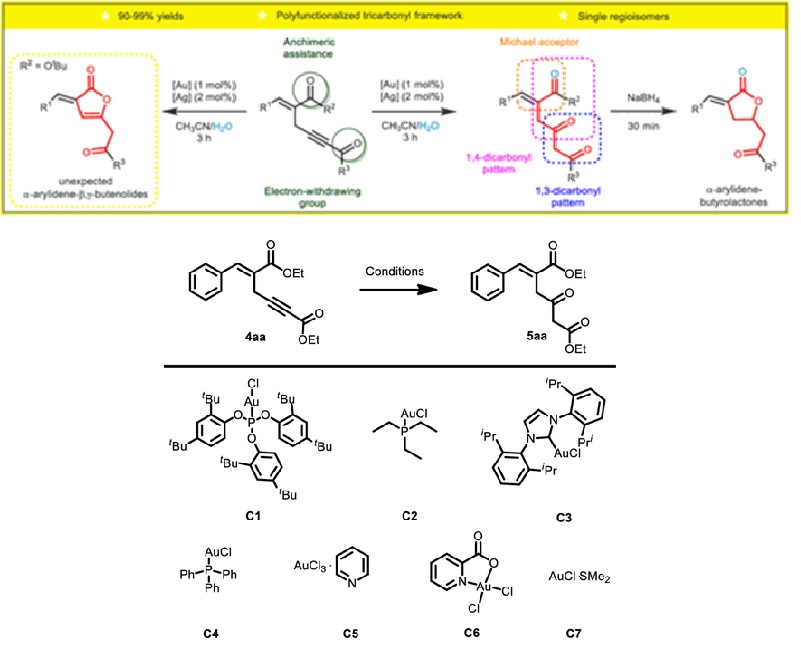
We report a direct, straightforward, and regioselective hydration of 1,4-enynes designed from Morita-Baylis- Hillman adducts. Under smooth conditions and short reaction times, gold-catalyzed hydration of internal alkynes provides synthetically useful ketones as single regioisomers in yields higher than 90%. The synthetic usefulness of this protocol was demonstrated by the conversion of selected ketones into biologically valuable alpha-alkylidene-gamma-lactones upon reduction with sodium borohydride. In the course of the scope evaluation, we discovered that this methodology could also furnish alpha-arylidene-beta,gamma-butenolides.
1 Laboratory of Synthesis of Natural Products and Drugs, Institute of Chemistry, University of Campinas, UNICAMP, P.O. Box 6154, 13083-970, Campinas, SP, Brazil
Link to article: https://pubs.acs.org/doi/10.1021/acsomega.0c00101