By Rui Sintra, Assessoria de Comunicação of IFSC
A molecule located in the Plasmodium falciparum system – main parasite that causes malaria – can alert this organism of possible dangers. By the way, when it has interrupted its activity, it can cause the death of the parasite. These facts, which may be taken as some of the “keys” of the discovery of the fight against malaria, were observed by researchers at the Center for Research and Innovation in Biodiversity and Drug Discovery (CIBFar-CEPID) of the São Carlos Institute of Physics (IFSC) through a study carried out in partnership with specialists of the Institute of Biosciences (IB), both from USP. The referring article to the discoveries was published last February in Scientific Reports of Nature. The CIBFar is a Center of Research, Innovation and Diffusion (CEPID) supported by the São Paulo Research Foundation (FAPESP).

Responsible for approximately 600,000 deaths per year, where children under five years account for approximately 78% of this index, malaria is an infectious tropical disease transmitted to humans through the bite of the female Anopheles mosquito. It is believed that one person dies from malaria in the world every minute. Moreover, it is estimated that three billion people are at risk of being affected by the disease, which can cause chills, high fever, headaches, muscle aches, delirium, drowsiness, vomiting, among other symptoms. In Brazil, the most common parasite that causes the disease is Plasmodium vivax. It hardly takes the patient to death, but cause great morbidity. Plasmodium falciparum, cited above, is the most feared, due to be the main cause of deaths from malaria in the world.
When the P. falciparum invades the bloodstream, it lodges in red blood cells (RBCs), and to obtain enough energy and survive in the human body, it degrades hemoglobin, which are the major proteins present in RBC, which are responsible for red coloration of blood.
Although seem simple, the process of obtaining energy is complex, since within these proteins, there is a molecule called “heme” that is released when the parasite degrades hemoglobin, being soluble in red blood cells and becoming toxic to Plasmodium. However, throughout its evolution, P. falciparum “learned” to protect against this toxic molecule, polymerizing it and precipitating it. “The parasite can not handle the heme; then he leaves this molecule ‘aside’. It bothers the parasite, but is no longer toxic to him”, explains Professor Rafael Guido, professor of Group of Crystallography of IFSC, who was part of this work, whose main author was divided among researchers Fernando Maluf of IFSC and Eduardo Alves of IB.
“Detoxification”
This was just one of the ways that P. falciparum found over the years to dodge the heme and survive in the host. One alternative used by the parasite is known as “detoxification” process wherein the P. falciparum transforms the heme group into a molecule called biliverdin, through heme oxygenase, a molecule present in both the human body and the parasite system, which oxidizes the heme group.
After 72 hs within a hemoglobin, the parasite multiplies a lot causing the explosion of the cell and release of the parasites in the human body, which may cause complications to the patient, for example, bleeding (sudden loss of blood that can lead to death).
During some studies done in the laboratory, professor Celia Garcia, researcher at the IB and corresponding author of the paper, noted that P. falciparum produces biliverdin that, as the heme group, can be toxic for it. And, if this organism produces biliverdin, it is because there is some enzyme in its system that allows that. “In literature, there are many discussions about the enzyme be capable of converting heme into biliverdin in parasite system. But we found that there is biliverdin in this organism”, explains Guido, noting that the researchers came to this conclusion because they had found biliverdin in red blood cells where the parasite had installed. In these blood cells, do not exist human enzymes capable of producing biliverdin, so in that case it can only have been produced by the parasite.
At this stage of the study, the researchers nocautearam (knocked out – common term used in scientific circles) the parasite, that is, had removed the gene encoding heme oxygenase enzyme thus preventing the production of biliverdin. When the parasite was knocked out, he died. “Having removed the parasite gene is lethal to it. This is very interesting for us, we want to develop a drug to combat malaria, because it shows that if we prevent the production or action of heme oxygenase [that molecule which oxidizes the heme group], we can combat the parasite”, says Guido.
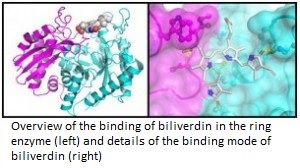
Moreover, it was observed that biliverdin modifies the parasite’s life cycle, preventing the development and maturation of P. falciparum. According to the professor of IFSC, if it is so important, exists some another protein that binds to biliverdin. But then, which would be this protein? Based on this question, the researchers developed another series of laboratory tests, which found that enolase enzyme joins to biliverdin, being an important enzyme for the parasite. This discovery surprised researchers, as enolase is an enzyme which is located in another way of Plasmodium organism and in principle would not have to join to the group heme.
After the discovery, the enolase was cloned, expressed and purified – processes commonly performed to study an enzyme. From these procedures, researchers were able to characterize the interaction of biliverdin with the enzyme in question and concluded that biliverdin binds to enolase to alert the parasite of some danger. “The biliverdin would act as a communication molecule and enolase as the sensor which detects biliverdin. So if there is something wrong that can damage the parasite, it learns and protect itself by stopping the multiplication rate and keeping more resistant forms”, says Guido
After this series of studies that reveal such discoveries “unexpected and curious, opening a frontier for many other works aimed at combating malaria”, the researchers are investigating the enolase, in order to get inhibitors that could prevent the interaction of biliverdin with this enzyme, as well as its natural function, making the parasite an easier target to be fought in the future. Such phase of this research will be the focus of the next article by Guido and his fellow researchers.
In search of drugs
Despite having achieved such results, Guido explained that new drugs for malaria will become a reality in about one or two decades. However, the professor highlights another initiative of IFSC, which aims to develop new treatments for malaria: Soon, the Institute will have to establish, through professors Rafael Guido and Glaucius Oliva, a partnership with the non-governmental organization Medicines for Malaria Venture (Geneva, Switzerland). The purpose of this collaboration will be the development of new drug candidates aimed at combating malaria.
Photos: Divulgação / Scientists Against Malaria and IFSC
Source: Agência USP de Notícias