By José Tadeu Arantes from Agência FAPESP
A new compound that inhibits the replication of hepatitis C virus (HCV) at various stages of its cycle – and is capable of acting on bacteria, fungi and cancer cells – has been synthesized by researchers at the State University of São Paulo – Unesp.
The study – supported by FAPESP through various research promotion tools [see the report below] – was described in an article published in the journal Scientific Reports of the Nature group.
 “What we did was to combine existing molecules, through laboratory synthesis, to produce new compounds with biological potential. This method is called bioconjugation. By means of bioconjugation, we synthesized six compounds and tested them on HCV genotypes 2a and 3a. And we managed to reach a compound with great therapeutic potential”, said chemist Paulo Ricardo da Silva Sanches, one of the two main authors of the study, to Agência FAPESP.
“What we did was to combine existing molecules, through laboratory synthesis, to produce new compounds with biological potential. This method is called bioconjugation. By means of bioconjugation, we synthesized six compounds and tested them on HCV genotypes 2a and 3a. And we managed to reach a compound with great therapeutic potential”, said chemist Paulo Ricardo da Silva Sanches, one of the two main authors of the study, to Agência FAPESP.
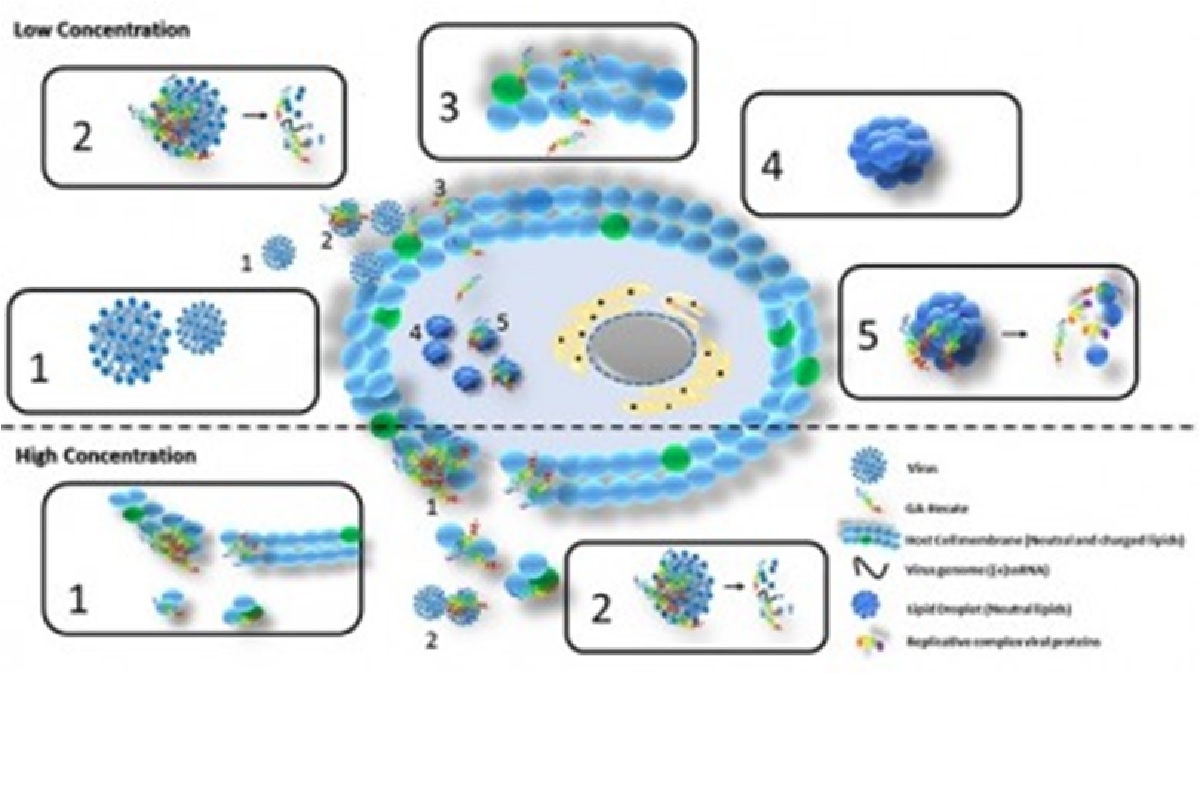
The hepatitis C virus presents significant genomic variability, exhibiting at least six major genotypes, each with subtypes. Genotypes 2a and 3a are the most common subtypes of circulating HCV. The compound capable of destroying them – AG-hecate – was synthesized from gallic acid and the hecate peptide.
“We found that this compound works at almost every stage of the HCV replicative cycle – which is not a common feature in antivirals. These usually have single, isolated targets such as capsid proteins, membrane receptors or specific proteins such as NS3, inhibiting specific processes such as virus entry into cells, synthesis of genetic and protein material, assembly and release of new particles viral infections. The AG-hecate, on the other hand, presented wide activity, acting in several stages of the cycle”, explained Sanches.
“The compound also has activity in the so-called ‘lipid droplets’ – lipid droplets inside which the virus circulates in cells and protects it from attack by enzymes. AG-hecate depletes these lipid droplets and leaves the replicative complex of the virus exposed to the action of cellular enzymes”, he continued.
The researchers tested AG-hecate in both the whole virus and so-called “subgenomic replicons”, which have all the elements for the replication of virus genetic material in cells, but are unable to synthesize proteins responsible for the infection. And the compound was efficient in all tests.
Another virtue presented by the compound was its high selectivity index. This means that it attacks the virus much more than the host cell. And thus, it has potential to be used as a drug in the treatment of the disease.
“Although the compound shows little activity in red blood cells, the molecule needs to undergo changes in its structure to further reduce its toxicity. That is what we are working on now, so that the research can evolve from the in vitro phase to the in vivo phase”, said the Unesp researcher.
 As reported by professor Eduardo Maffud Cilli, doctoral advisor of Sanches at the Institute of Chemistry of Unesp in Araraquara (SP), “the average time for the planning and development of therapeutic peptides is 10 years. A study with these data has just come out. So far, approximately two years have been spent on the development of the AG-hecate molecule”. “Considering the statistical average, it will take another eight years before the drug reaches the market”.
As reported by professor Eduardo Maffud Cilli, doctoral advisor of Sanches at the Institute of Chemistry of Unesp in Araraquara (SP), “the average time for the planning and development of therapeutic peptides is 10 years. A study with these data has just come out. So far, approximately two years have been spent on the development of the AG-hecate molecule”. “Considering the statistical average, it will take another eight years before the drug reaches the market”.
Cilli participated in the study and also signs the article published in Scientific Reports. “The great news is that this molecule does not only work on HCV. It can also act on bacteria, fungi and cancer cells. In addition, since zika and yellow fever viruses have very similar replicative cycles to HCV, we will also test the effectiveness of AG-hecate in relation to these viruses”, he said.
In the case of cancer, the molecule interacts and destroys the membrane of the affected cell. Here, the selectivity of AG-hecate action is due to the fact that the cancer-modified cell has a greater amount of negative charges on the surface than the normal cell. And the peptide has a positive charge. Then, the action is by electrostatic attraction. In the case of the virus, the mechanism of action of the molecule is more complex, as shown in the illustration.
The studies were carried out at the Laboratory of Synthesis and Biomolecular Studies of the Chemistry Institute of Unesp in Araraquara, coordinated by Professor Eduardo Maffud Cilli, and at the Laboratory of Genomic Studies of the Institute of Biosciences, Letters and Exact Sciences of Unesp in São José do Rio Preto, coordinated by Professor Paula Rahal, director of the doctorate of Mariana Nogueira Batista, researcher who divides the authorship of this work with Sanches.
Support from FAPESP

In addition to Sanches and Cilli, they participated in the study Mariana Nogueira Batista, Bruno Moreira Carneiro, Ana Cláudia Silva Braga, Guilherme Rodrigues Fernandes Campos and Paula Rahal.

The research was supported by FAPESP within the scope of the Center for Research and Innovation in Biodiversity and Drugs Discovery (CIBFar), one of the Research, Innovation and Dissemination Centers (CEPIDs) financed by FAPESP. The Foundation has also awarded fellowships to the following projects:
“Development of multifunctional pro-drugs for combination therapy against hepatocellular carcinoma and HCV.”
“Evaluation of synthetic peptides in targeted inhibition of hepatitis C virus”.
“Adaptation of an hcv-related rat derived rodent Hepacivirus to the murine host”
The article GA-Hecate antiviral properties on HCV whole cycle represent the new antiviral class and open the door for the development of broad spectrum antivirals can be read at http://www.nature.com/articles/s41598-018-32176-w
Full article: http://agencia.fapesp.br/cientistas-da-unesp-sintetizam-molecula-capaz-de-eliminar-o-virus-da-hepatite-c/29182/