Authors:
Silveira, Flavia F. [1, 2] ; de Souza, Juliana O. [3] ; Hoelz, Lucas V. B. [2] ; Campos, Vinicius R. [4] ; Jabor, Valquiria A. P. [5] ; Aguiar, Anna C. C. [3] ; Nonato, M. Cristina [5] ; Albuquerque, Magaly G. [1] ; Guido, Rafael V. C. [3] ; Boechat, Nubia [1, 2] ; Pinheiro, Luiz C. S. [2]
Abstract:
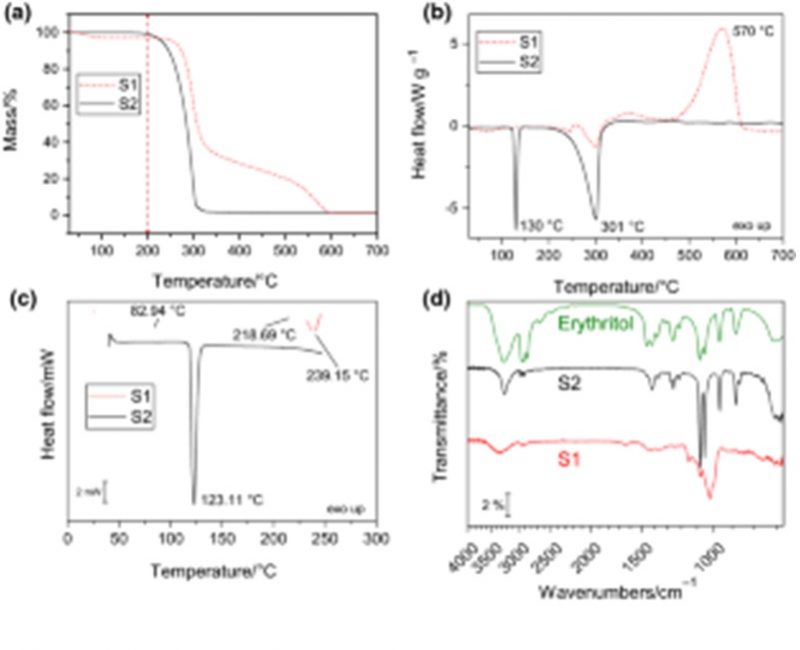
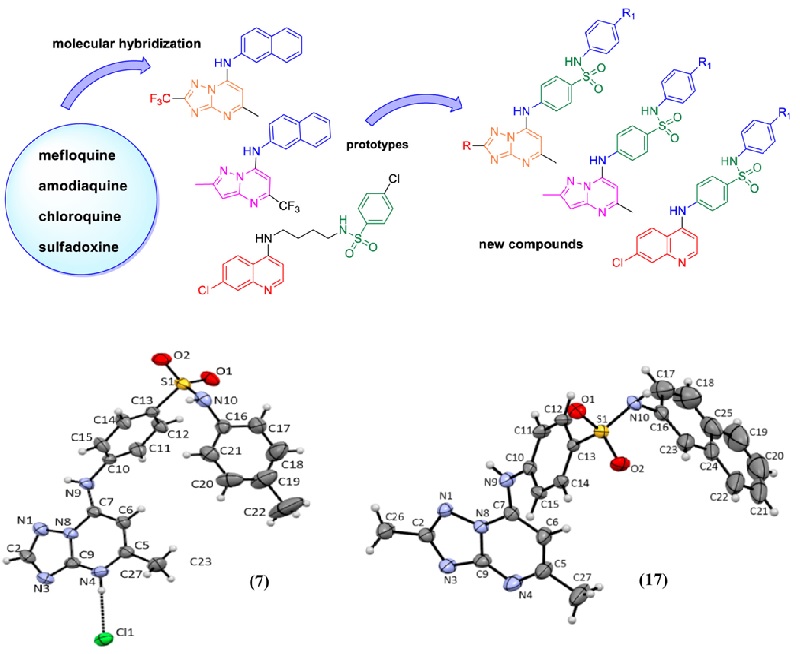
n this work, we designed and synthesized 35 new triazolopyrimidine, pyrazolopyrimidine and quinoline derivatives as P. falciparum inhibitors (3D7 strain). Thirty compounds exhibited anti-P. falciparum activity, with IC50 values ranging from 0.030 to 9.1 mu M. The {[}1,2,4] triazolo{[}1,5-a]pyrimidine derivatives were more potent than the pyrazolo{[}1,5-a]pyrimidine and quinoline analogues. Compounds 20, 21, 23 and 24 were the most potent inhibitors, with IC50 values in the range of 0.030-0.086 mu M and were equipotent to chloroquine. In addition, the compounds were selective, showing no cytotoxic activity against the human hepatoma cell line HepG2. All {[}1,2,4]triazolo{[}1,5-a]pyrimidine derivatives inhibited PfDHODH activity in the low micromolar to low nanomolar range (IC50 values of 0.08-1.3 mu M) and did not show significant inhibition against the HsDHODH homologue (0-30% at 50 mu M). Molecular docking studies indicated the binding mode of {[}1,2,4]triazolo{[}1,5-a]pyrimidine derivatives to PfDHODH, and the highest interaction affinities for the PfDHODH enzyme were in agreement with the in vitro experimental evaluation. Thus, the most active compounds against P. falciparum parasites 20 (R = CF3, R-1 = F; IC50 = 0.086 mu M), 21 (R = CF3; R-1 = CH3; IC50 = 0.032 mu M), 23, (R = CF3, R-1 = CF3; IC50 = 0.030 mu M) and 24 (R = CF3, 2-naphthyl; IC50 = 0.050 mu M) and the most active inhibitor against PfDHODH 19 (R = CF3, R-1 = Cl; IC50 = 0.08 mu M – PfDHODH) stood out as new lead compounds for antimalarial drug discovery. Their potent in vitro activity against P. falciparum and the selective inhibition of the PfDHODH enzyme strongly suggest that this is the mechanism of action underlying this series of new {[}1,2,4]triazolo{[}1,5-a]pyrimidine derivatives. (c) 2020 Elsevier Masson SAS. All rights reserved.
1 Laboratorio de Sintese de Farmacos, Instituto de Tecnologia em Farmacos, Farmanguinhos – FIOCRUZ, Fundacao Oswaldo Cruz. Rua Sizenando Nabuco 100, Manguinhos, Rio de Janeiro, RJ, 21041-250, Brazil
2 Programa de Pós-Graduação em Química, PGQu Instituto de Química, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
3 Instituto de Física de São Carlos, Universidade de São Paulo, Av. João Dagnone, 1.100, Jd. Santa Angelina, São Carlos, SP, Brazil
4 Departamento de Química Orgânica, Programa de Pós-Graduação em Química, Instituto de Química, Universidade Federal Fluminense, Niterói, RJ, Brazil
5 Laboratório de Cristalografia de Proteínas, Departamento de Ciências BioMoleculares, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Avenida do Café s/n Monte Alegre, 14040-903, Ribeirão Preto, SP, Brazil
Link to article: https://www.sciencedirect.com/science/article/abs/pii/S0223523420309132?via%3Dihub