Authors:
Agy, Andre Capretz 1 ; Rodrigues, Jr., Manoel T. 1 ; Zeoly, Lucas A. 1 ; Simoni, Deborah A. 2 ; Coelho, Fernando 1
Abstract:
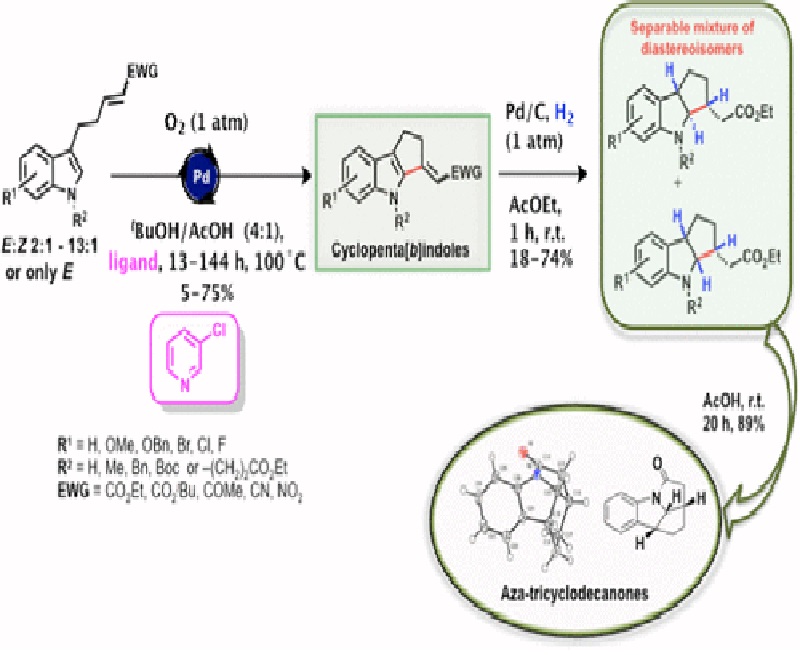
The cyclopenta[b]indole moiety represents a key skeletal unit in several natural and synthetic compounds that exhibit diverse biological properties. We described herein a two-step sequence for synthesizing cyclopenta[b]indoles with great structural diversity in overall yields up to 37%. The key step was a palladium-catalyzed oxidative annulation of 3-alkylindoles (Fujiwara–Moritani reaction). The obtained cyclopenta[b]indoles were used as substrates in heterogeneous hydrogenation reactions to afford new fused indolines in moderate yields. An acid-catalyzed intramolecular cyclization of three such indolines gave tetracyclic lactams in 89, 90, and 61% yields.
1 University of Campinas, Institute of Chemistry, Laboratory of Synthesis of Natural Products and Drugs, P.O. Box 6154, 13083-979 Campinas, SP, Brazil
2 University of Campinas, Institute of Chemistry, Laboratory of Crystallography, P.O. Box 6154, 13083-979 Campinas, SP, Brazil
Link to article: https://pubs.acs.org/doi/10.1021/acs.joc.9b00505